A System Releases 622 Kj Of Heat
A system releases 622 kj of heat. The gas in a piston defined as the system warms and absorbs 655 J. What is the change in internal energy of the system. So were looking at the change in internal energy off the system.
So from this problem we have to take our words here and use those to determine the signs in front of our Q and W. If a system releases 622 kJ of heat and does 1005 kJ of work on the surroundings. Get the detailed answer.
Its what we are looking for Change in any way about copulate. A System releases 100 kJ of heat with is done - 23954322 siddharthbhosale305 siddharthbhosale305 27092020 Math Secondary School answered A System releases 100 kJ of heat with is done on the system calculate change in internal energy 2. So cue if it wants to minors.
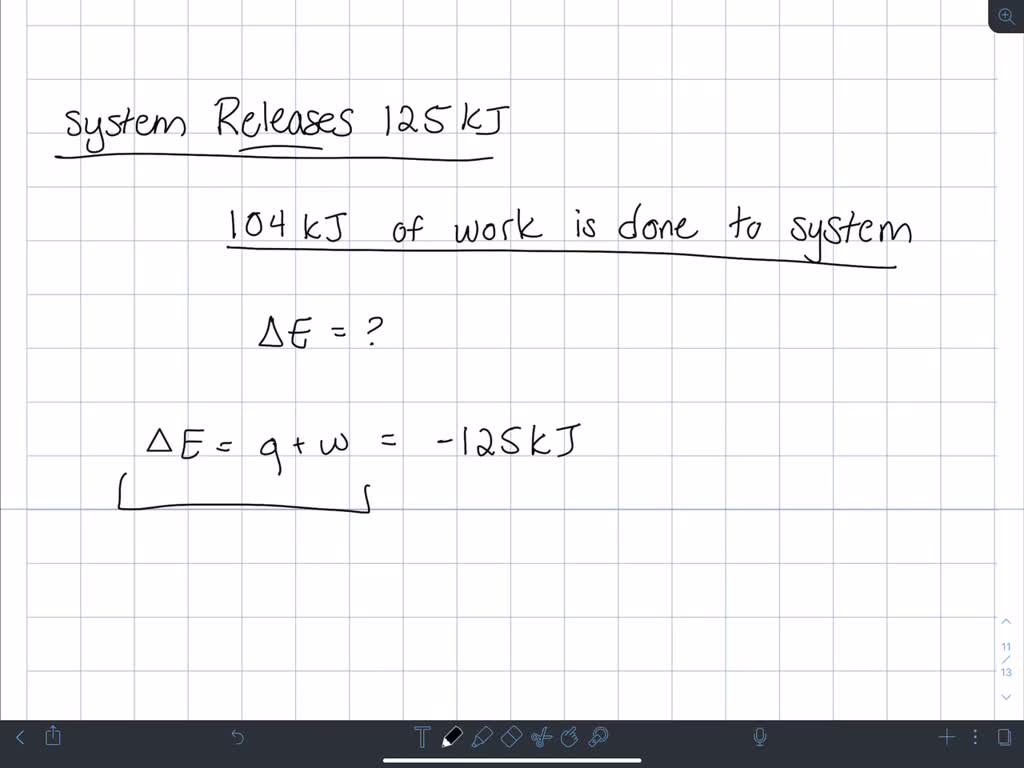
Which makes them negative. A system releases 622 mathrmkJ of heat and does 105 mathrmkJ of wo 0051 A system absorbs 215 mathrmkJ of heat and 116 mathrmkJ of work. In this question were told or ah system.
What is the change in internal energy of the system. What is the change in the internal energy of the system. Which of the following statements is false.
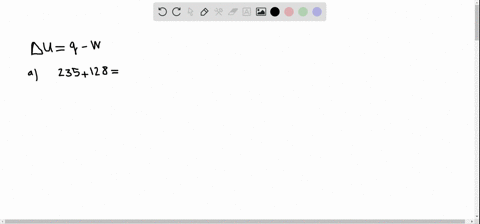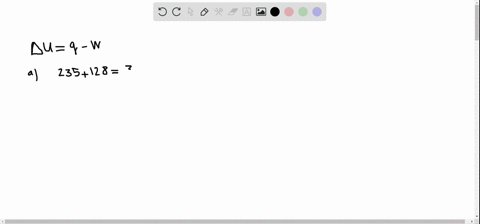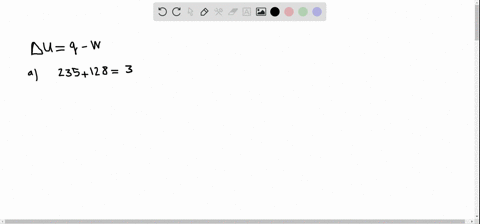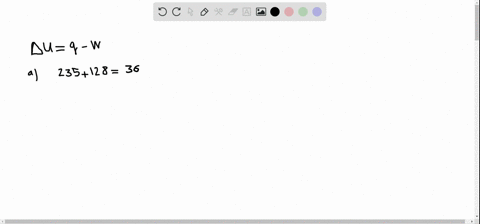
A system releases 622 kJ of heat and does 105 kJ of work on the surroundings. A system releases 415 kJ of heat a. 622kJ are released by the system and 105kJ of work are done by the system on the surroundings.
So if the system is absorbing heat energy were gonna have a positive que so positive 45. What is the change in internal energy of the system.
Which of the following statements is false.
Get the detailed answer. Both indicate a loss of energy by the system. A system releases 622 kJ of heat and does 105 kJ of work on the surroundings. Internal Energy Video Lessons. What is the change in internal energy of thesystem. And it does 29 killed Jules of work on the surroundings. Work done by the system on surroundings is 105 kJ as work is done by the system it is negative. A Heat flows from the system to the surroundings B The. Which of the following statements is false.
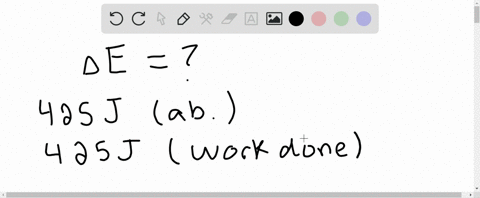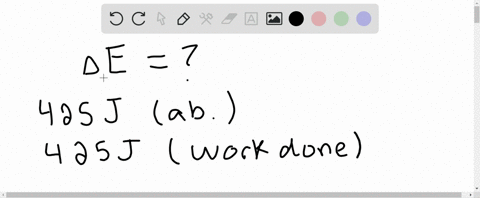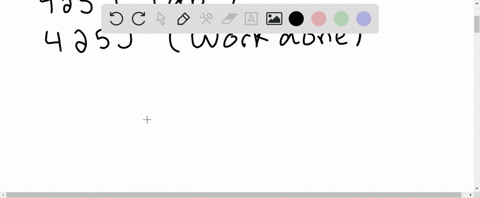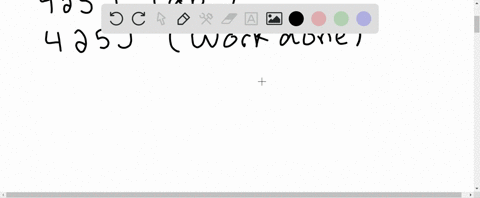
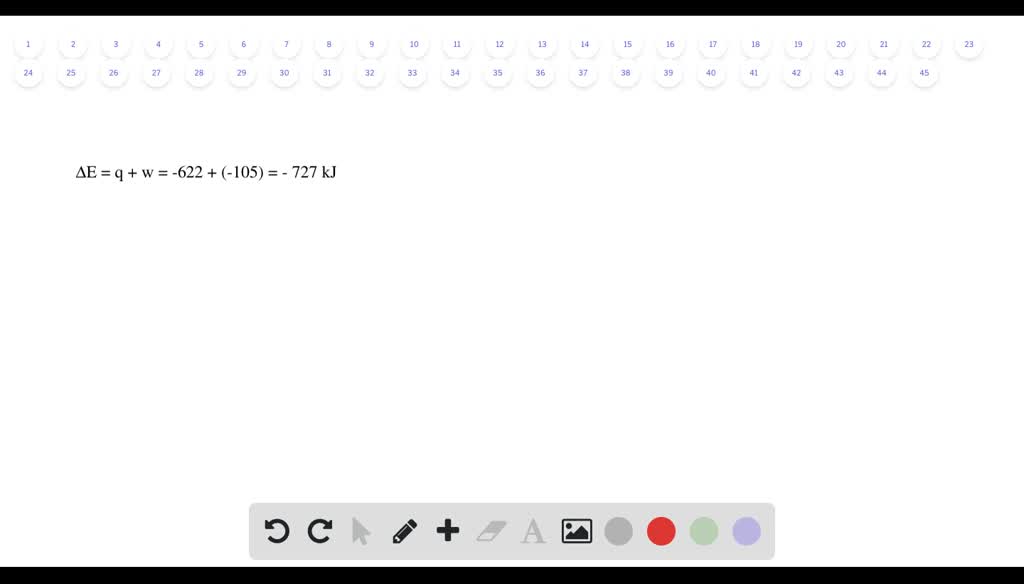
Six Thio kill germs on it. Which makes them negative. Were gonna be looking at it system of gas on this system absorbs 45 killer jewels of energy in the form of heat. Solution for A system releases 622 kJ of heat and does 105 kJ of work on thesurroundings. What is the change in internal energy of the system. Six Thio kill germs on it. ΔE q w q -622 kJ -622kJ -105kJ -727 kJ.
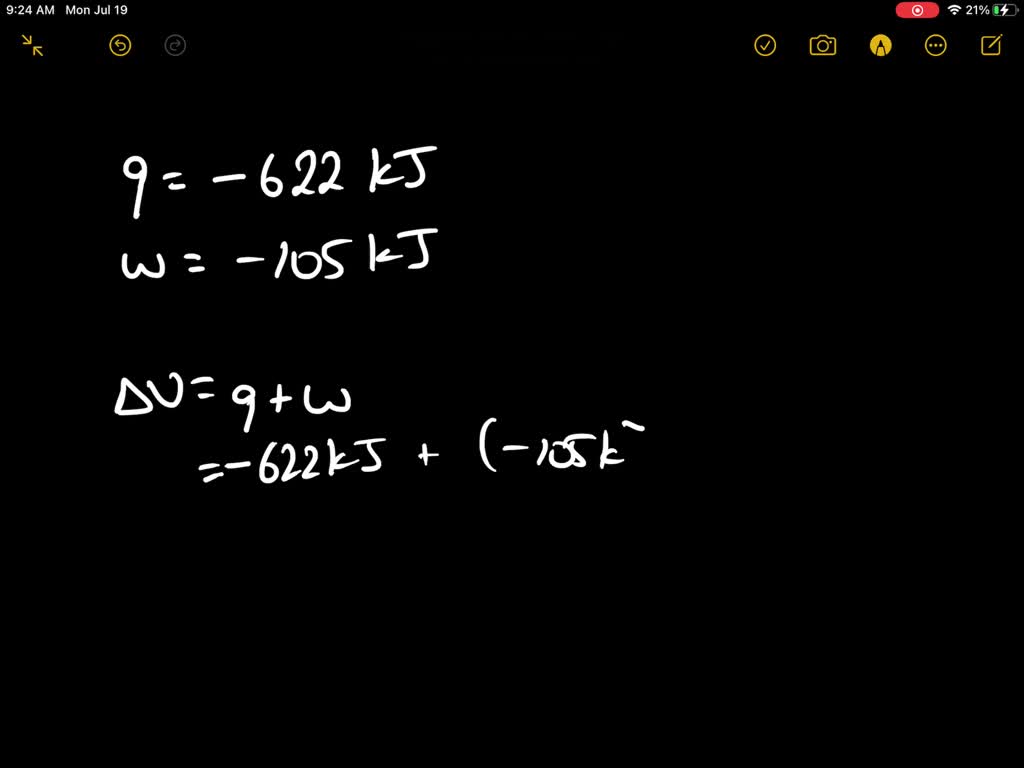


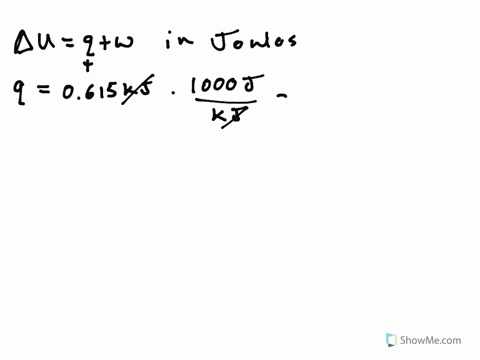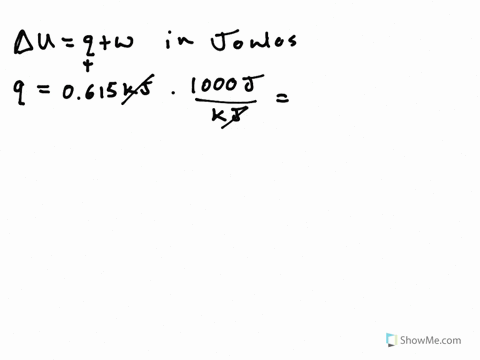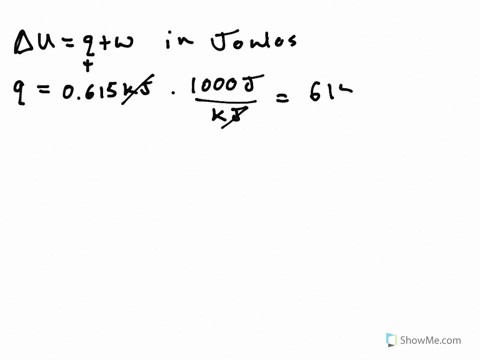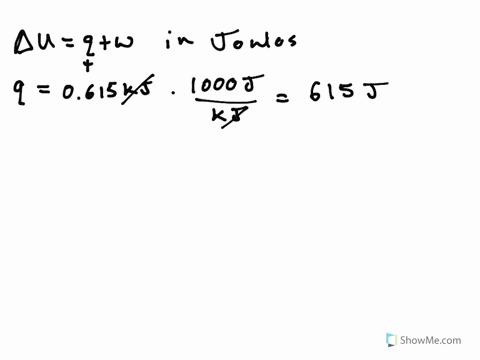




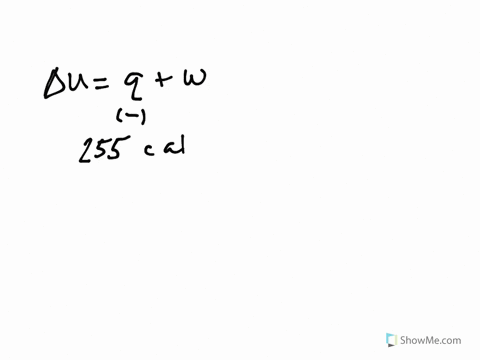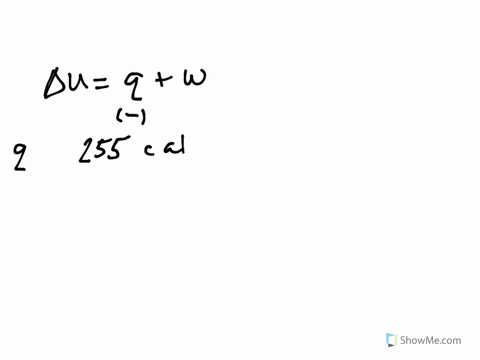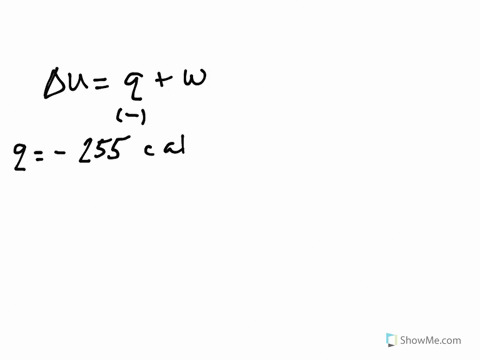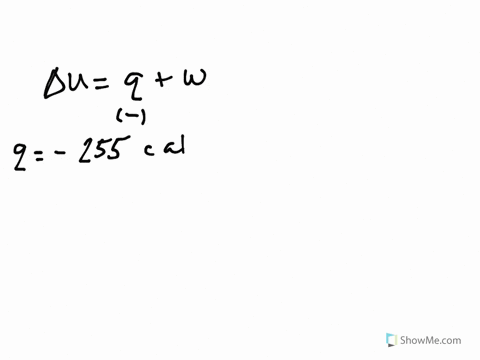


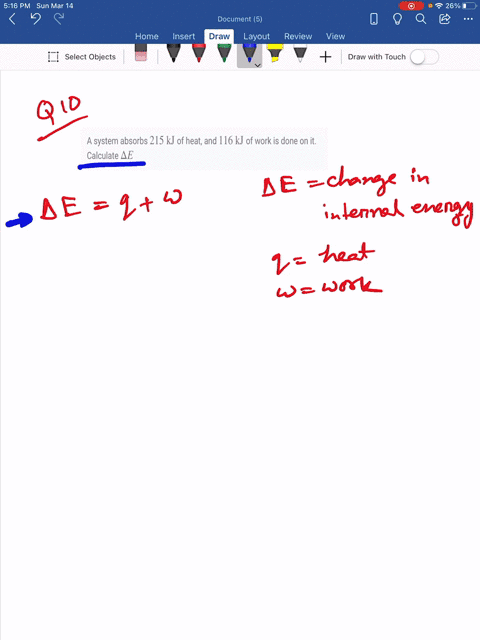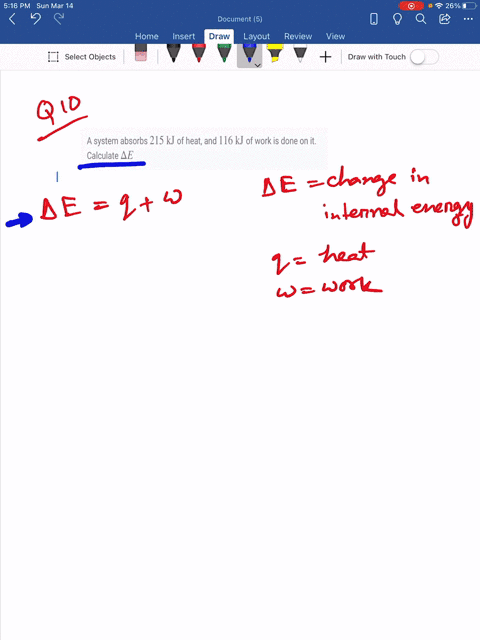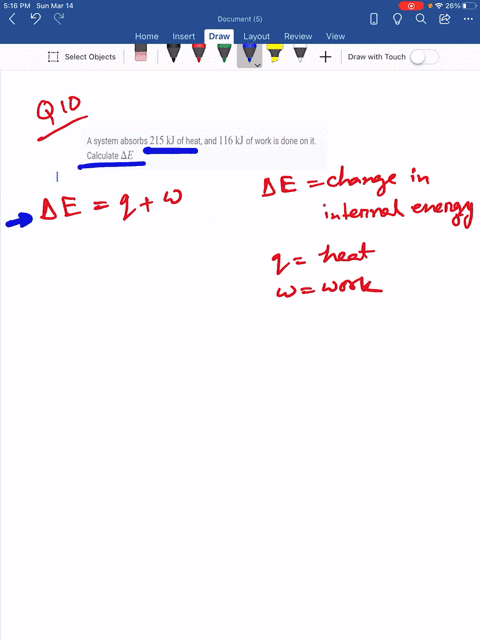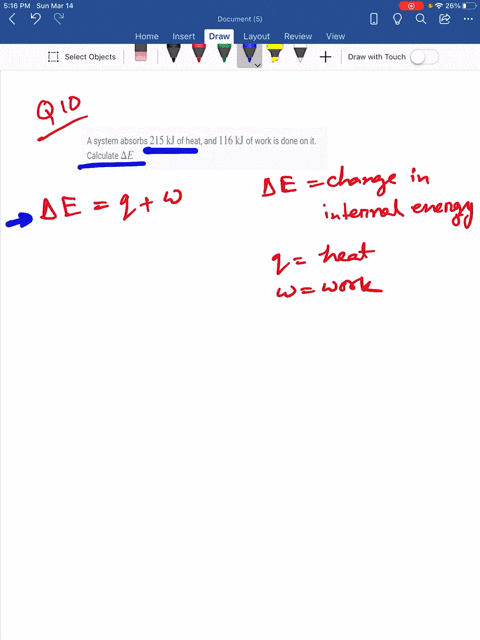













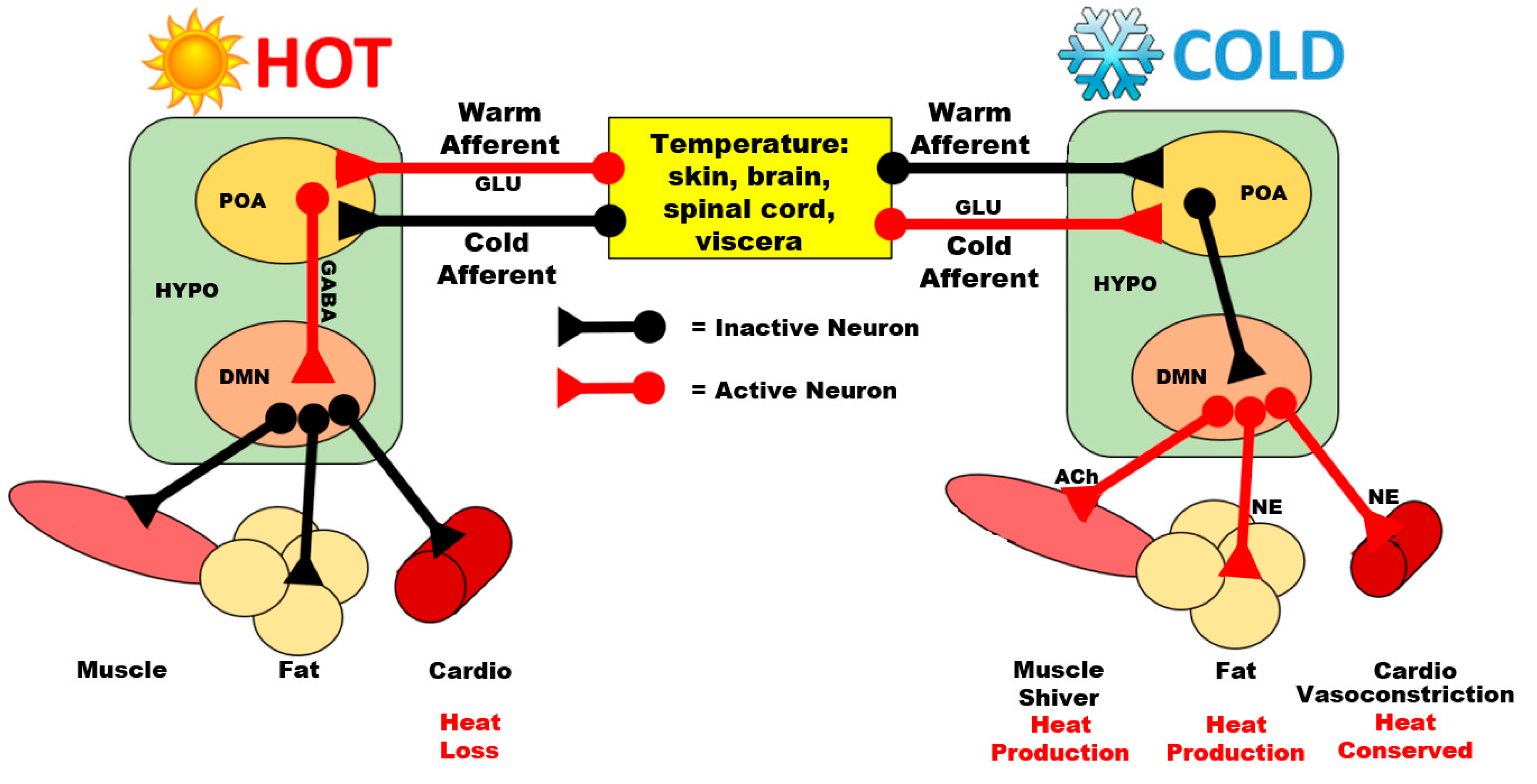
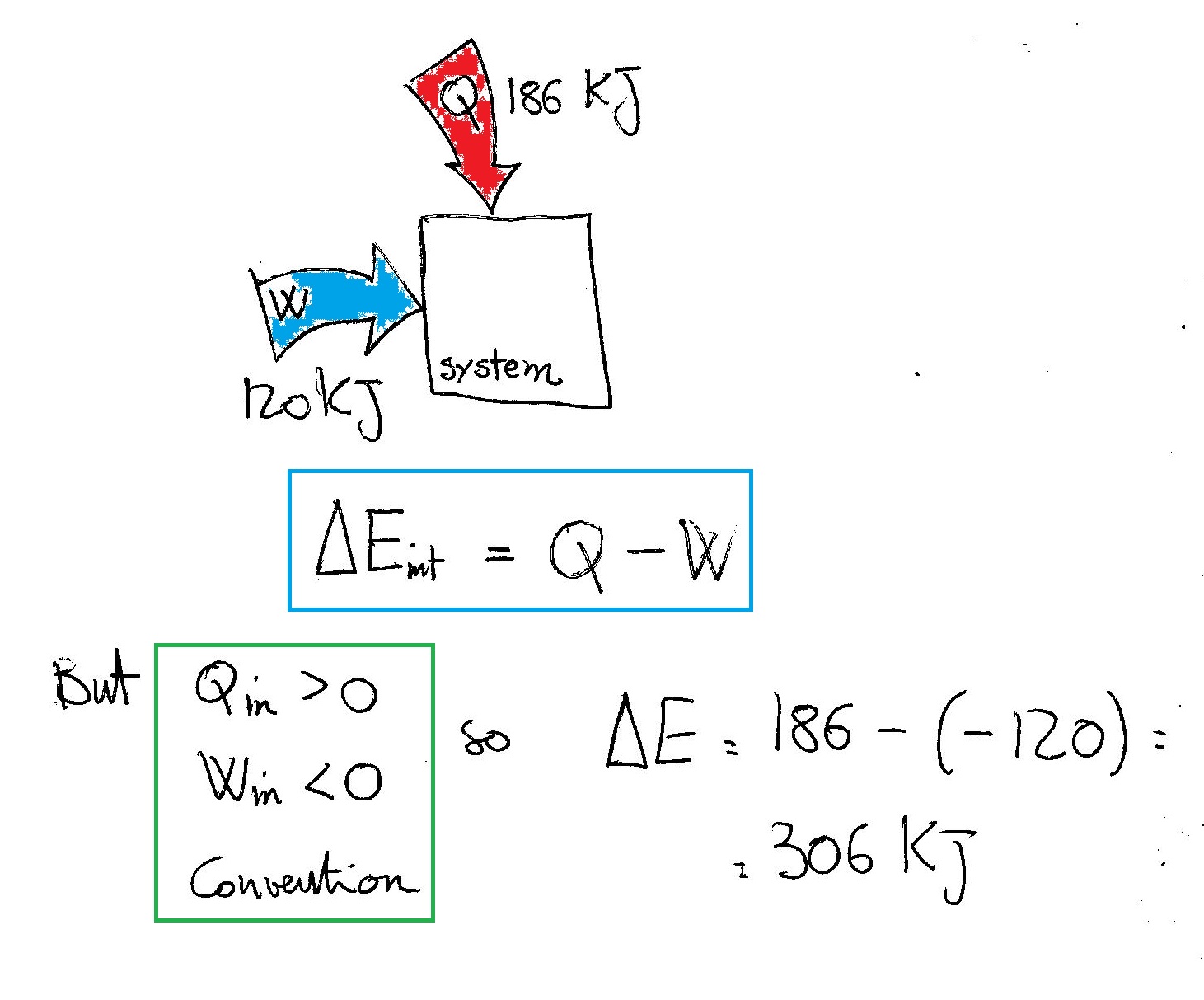








Post a Comment for "A System Releases 622 Kj Of Heat"